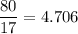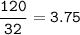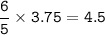Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

You know the right answer?
In a lab experiment 80.0 g of ammonia [NH3] and 120 g of oxygen are placed in a reaction vessel. At...
Questions





Social Studies, 29.07.2019 16:40

Social Studies, 29.07.2019 16:40




Mathematics, 29.07.2019 16:40



Chemistry, 29.07.2019 16:40




History, 29.07.2019 16:40

Physics, 29.07.2019 16:50

Geography, 29.07.2019 16:50