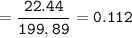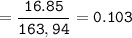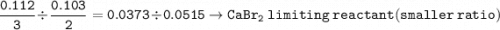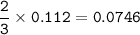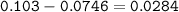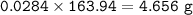Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 23.06.2019 05:30
Stoichiometry- i need with 14 and 15! an explanation would be appreciated!
Answers: 1
You know the right answer?
A mixture of 22.44 g of CaBr2 and 16.85 g Na3PO4 is used in the following reaction. Determine the ma...
Questions

Chemistry, 16.11.2020 20:20

Mathematics, 16.11.2020 20:20



Health, 16.11.2020 20:20

Health, 16.11.2020 20:20

Mathematics, 16.11.2020 20:20

Biology, 16.11.2020 20:20

Mathematics, 16.11.2020 20:20

Mathematics, 16.11.2020 20:20

Mathematics, 16.11.2020 20:20


Mathematics, 16.11.2020 20:20

Mathematics, 16.11.2020 20:20



Mathematics, 16.11.2020 20:20


Mathematics, 16.11.2020 20:20