
Chemistry, 06.12.2020 07:20 elitehairnerd1964
TNT is manufactured by the reaction of toluene with nitric acid according to the following equation: C7H8 (l) + 3 HNO3 (aq) ---> C7H5(NO2)3 (s) + 3 H2O(l) Calculate the mass of TNT expected from the reaction of 69.0 g of toluene

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Using complete sentences, explain how to predict the products and balance the reaction between sulfuric acid and potassium hydroxide.
Answers: 1

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
TNT is manufactured by the reaction of toluene with nitric acid according to the following equation:...
Questions


Social Studies, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10


Mathematics, 18.03.2021 03:10



History, 18.03.2021 03:10



Mathematics, 18.03.2021 03:10

English, 18.03.2021 03:10

Health, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10




History, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10

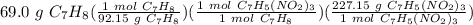 = 170.085 g C₇H₅(NO₂)₃
= 170.085 g C₇H₅(NO₂)₃

