
Chemistry, 06.12.2020 14:00 cthompson1107
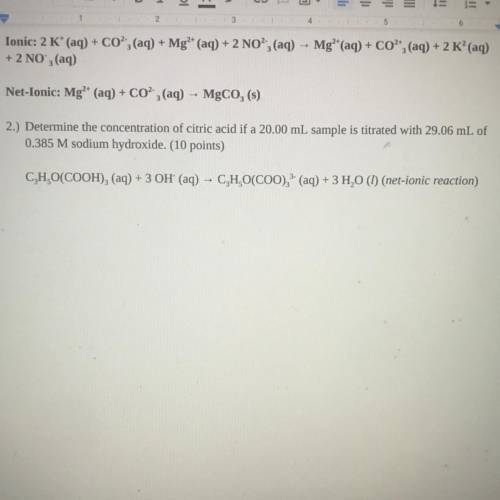
2.) Determine the concentration of citric acid if a 20.00 mL sample is titrated with 29.06 mL of
0.385 M sodium hydroxide. (10 points)
C, H,O(COOH)2 (aq) + 3 OH' (aq) - C, H,O(COO), (aq) + 3 H20 (1) (net-ionic reaction)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
2.) Determine the concentration of citric acid if a 20.00 mL sample is titrated with 29.06 mL of
0....
Questions


Mathematics, 15.04.2020 19:59




Social Studies, 15.04.2020 20:00

Chemistry, 15.04.2020 20:00


Physics, 15.04.2020 20:00


Mathematics, 15.04.2020 20:00

History, 15.04.2020 20:00

Mathematics, 15.04.2020 20:00









