
Chemistry, 06.12.2020 19:00 alanflores40
The pH of a 0.15 M butylamine, C&H3NH2 solution is 12.0 at 25°C. Calculate the dissociation constant of the base.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1
You know the right answer?
The pH of a 0.15 M butylamine, C&H3NH2 solution is 12.0 at 25°C. Calculate the dissociation
con...
Questions


Computers and Technology, 26.08.2021 16:40



Mathematics, 26.08.2021 16:40


History, 26.08.2021 16:40






Mathematics, 26.08.2021 16:40

English, 26.08.2021 16:40



Mathematics, 26.08.2021 16:40

Mathematics, 26.08.2021 16:40


Biology, 26.08.2021 16:40

![\rm Kb=\dfrac{[L][OH^-]}{[LOH]}](/tpl/images/0954/6725/84d1f.png)
![\tt [OH^-]=\sqrt{Kb.M}](/tpl/images/0954/6725/ab388.png)
![\tt [OH^-]=10^{-pOH}\\\\(OH^-]=10^{-2}](/tpl/images/0954/6725/b2b50.png)
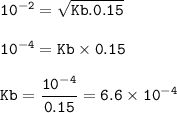
![\tt Kb=\dfrac{x^2}{0.15-x}\rightarrow x=[OH^-]\\\\Kb=\dfrac{10^{-4}}{0.15-10^{-2}}=7.14\times 10^{-4}](/tpl/images/0954/6725/69346.png)


