
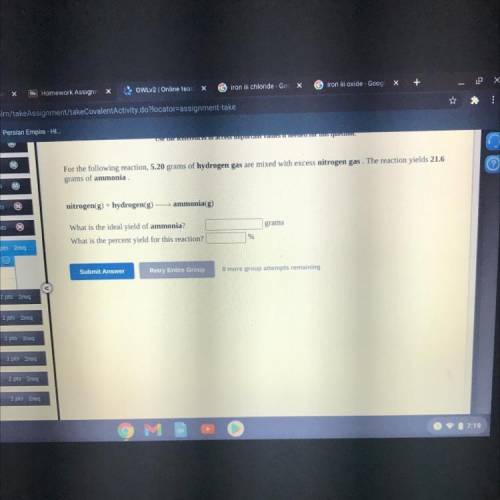
For the following reaction, 5.20 grams of hydrogen gas are mixed with excess nitrogen gas. The reaction yields 21.6
grams of ammonia
nitrogen(g) + hydrogen(g) — ammonia(g)
grams
What is the ideal yield of ammonia?
What is the percent yield for this reaction?
%
Submit Answer
Retry Entire Group
8 more group attempts remaining

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1


You know the right answer?
For the following reaction, 5.20 grams of hydrogen gas are mixed with excess nitrogen gas. The react...
Questions



Mathematics, 18.03.2021 01:10

Advanced Placement (AP), 18.03.2021 01:10


Mathematics, 18.03.2021 01:10




Mathematics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

Chemistry, 18.03.2021 01:10


Chemistry, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

Social Studies, 18.03.2021 01:10

Computers and Technology, 18.03.2021 01:10


English, 18.03.2021 01:10




