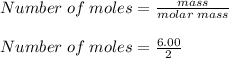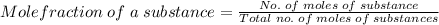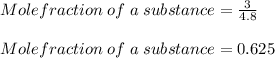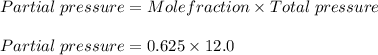Chemistry, 07.12.2020 06:00 RandomUser101
What is the partial pressure of hydrogen gas in a mixture that contains 6.00 grams of hydrogen gas and 1.80 mol carbon dioxide with a total pressure of 12.0 atm?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2
You know the right answer?
What is the partial pressure of hydrogen gas in a mixture that contains 6.00 grams of hydrogen gas a...
Questions


Computers and Technology, 14.06.2021 19:00


Social Studies, 14.06.2021 19:00

Mathematics, 14.06.2021 19:00


Spanish, 14.06.2021 19:00


Mathematics, 14.06.2021 19:00


Chemistry, 14.06.2021 19:00




Health, 14.06.2021 19:00

Mathematics, 14.06.2021 19:00


Mathematics, 14.06.2021 19:00

Mathematics, 14.06.2021 19:00

Mathematics, 14.06.2021 19:00