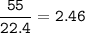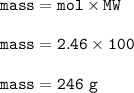Chemistry, 07.12.2020 06:00 nails4life324
When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reaction
CaCO3(s)→CaO(s)+CO2(g)
What is the mass of calcium carbonate needed to produce 55.0 L of carbon dioxide at STP?
Express your answer with the appropriate units.
mass of CaCO3 =

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2
You know the right answer?
When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reac...
Questions

Arts, 29.08.2019 19:30

Biology, 29.08.2019 19:30

Chemistry, 29.08.2019 19:30


Computers and Technology, 29.08.2019 19:30



Mathematics, 29.08.2019 19:30



Physics, 29.08.2019 19:30

Mathematics, 29.08.2019 19:30


History, 29.08.2019 19:30

Mathematics, 29.08.2019 19:30

Social Studies, 29.08.2019 19:30



Mathematics, 29.08.2019 19:30

French, 29.08.2019 19:30