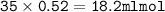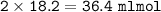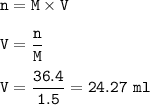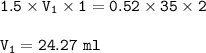Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
HELP In a neutralization reaction, aqueous HCl and aqueous Ba(OH)2 react to form aqueous BaCl2 and w...
Questions


World Languages, 28.08.2019 00:30

Mathematics, 28.08.2019 00:30




History, 28.08.2019 00:30


Biology, 28.08.2019 00:30


Geography, 28.08.2019 00:30


History, 28.08.2019 00:30




Mathematics, 28.08.2019 00:30

Mathematics, 28.08.2019 00:30

Chemistry, 28.08.2019 00:30

Mathematics, 28.08.2019 00:30