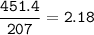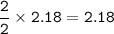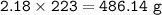Consider the reaction.
2Pb(s)+O2(g)⟶2PbO(s)
An excess of oxygen reacts with 451.4 g of...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3


Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
Questions

Mathematics, 26.08.2019 16:10

Mathematics, 26.08.2019 16:10

Mathematics, 26.08.2019 16:10


Physics, 26.08.2019 16:10


English, 26.08.2019 16:10


Business, 26.08.2019 16:10


History, 26.08.2019 16:10