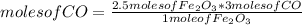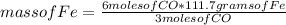Consider the process used to produce iron metal from its ore.
Fe2O3(s) + 3CO(g) --> 2Fe(s) + 3CO2 (g)
How many grams of iron can be produced from 2.5 moles of Fe2O3 and 6.0 moles of CO? Hint: limiting reactant problem
O A. 140 g
B. 335 g
C. 55.858
D. 223 g Fe
E. 279 g Fe

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2


Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1

Chemistry, 23.06.2019 05:30
Scientist think that animals with remarkably similar embryo development probably shared a common ancestor
Answers: 1
You know the right answer?
Consider the process used to produce iron metal from its ore.
Fe2O3(s) + 3CO(g) --> 2Fe(s) + 3CO...
Questions

Mathematics, 27.07.2019 14:00


French, 27.07.2019 14:00


Computers and Technology, 27.07.2019 14:00

Biology, 27.07.2019 14:00

Biology, 27.07.2019 14:00






Biology, 27.07.2019 14:10

Chemistry, 27.07.2019 14:10

Health, 27.07.2019 14:10