
Chemistry, 08.12.2020 02:10 jeanniebyrd54
A major component of gasoline is octane. When liquid octane is burned in air it reacts with oxygen gas to produce carbon dioxide gas and water vapor. Calculate the moles of octane needed to produce 2.40 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to 3 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

You know the right answer?
A major component of gasoline is octane. When liquid octane is burned in air it reacts with oxygen g...
Questions


Mathematics, 05.05.2020 00:04

Mathematics, 05.05.2020 00:04


Mathematics, 05.05.2020 00:04

English, 05.05.2020 00:04


Mathematics, 05.05.2020 00:04




Mathematics, 05.05.2020 00:04

English, 05.05.2020 00:04

Chemistry, 05.05.2020 00:04

Biology, 05.05.2020 00:04

English, 05.05.2020 00:04

Social Studies, 05.05.2020 00:04



Mathematics, 05.05.2020 00:04

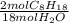 = 0.267 mol C₈H₁₈
= 0.267 mol C₈H₁₈

