
Chemistry, 08.12.2020 05:50 bbyniah123
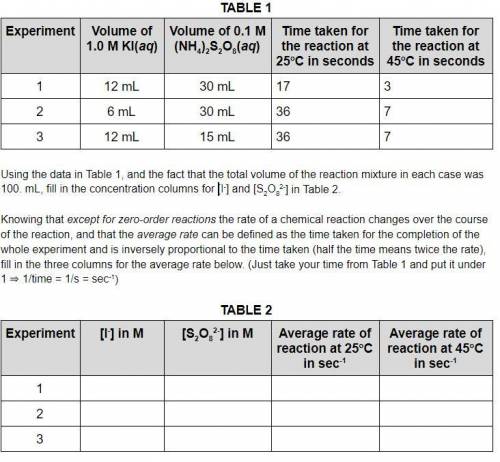
Using the data in Table 1, and the fact that the total volume of the reaction mixture in each case was 100. mL, fill in the concentration columns for [I-] and [S2O82-] in Table 2. Knowing that except for zero-order reactions the rate of a chemical reaction changes over the course of the reaction, and that the average rate can be defined as the time taken for the completion of the whole experiment and is inversely proportional to the time taken (half the time means twice the rate), fill in the three columns for the average rate below. (Just take your time from Table 1 and put it under 1 ⇒ 1/time = 1/s = sec-1)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
Using the data in Table 1, and the fact that the total volume of the reaction mixture in each case w...
Questions


Mathematics, 06.11.2019 05:31



History, 06.11.2019 05:31

English, 06.11.2019 05:31

History, 06.11.2019 05:31

Mathematics, 06.11.2019 05:31

Biology, 06.11.2019 05:31

Mathematics, 06.11.2019 05:31


Mathematics, 06.11.2019 05:31


English, 06.11.2019 05:31

Chemistry, 06.11.2019 05:31


Mathematics, 06.11.2019 05:31

Mathematics, 06.11.2019 05:31


Mathematics, 06.11.2019 05:31



