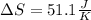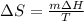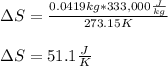Chemistry, 08.12.2020 14:00 choiboiqg5755
Calculate the change in entropy as 0.0419 kg of ice at 273.15 K melts. The latent heat of fusion of water is 333000 J/kg .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
Calculate the change in entropy as 0.0419 kg of ice at 273.15 K melts. The latent heat of fusion of...
Questions

Mathematics, 06.07.2019 07:30

Mathematics, 06.07.2019 07:30




Mathematics, 06.07.2019 07:30


Mathematics, 06.07.2019 07:30



German, 06.07.2019 07:30

Mathematics, 06.07.2019 07:30

Mathematics, 06.07.2019 07:30

Mathematics, 06.07.2019 07:30



English, 06.07.2019 07:30