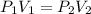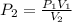Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2
You know the right answer?
If 489 mL of carbon dioxide has a pressure of 2.75 atm. If the gas is compressed to 254 mL, what is...
Questions


History, 08.01.2021 01:30

Mathematics, 08.01.2021 01:30



Mathematics, 08.01.2021 01:30

Mathematics, 08.01.2021 01:30

Spanish, 08.01.2021 01:30

Mathematics, 08.01.2021 01:30

Mathematics, 08.01.2021 01:30

Mathematics, 08.01.2021 01:30

Geography, 08.01.2021 01:30

Health, 08.01.2021 01:30


Mathematics, 08.01.2021 01:30



Mathematics, 08.01.2021 01:30


Spanish, 08.01.2021 01:30