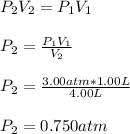A gas is initially confined to a 1.00 L vessel at 3.00 atm of pressure. A valve connecting the 1.00 L vessel to a 3.00 L vessel is opened and the gas expands. What is the final pressure in atm of the gas after the valve is opened, assuming that the volume of the connecting tube is negligible and there is no temperature change?
a. 0.250 atm
b. 0.500 atm
c. 0.750 atm
d. 1.00 atm
e. None of these choices

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
A gas is initially confined to a 1.00 L vessel at 3.00 atm of pressure. A valve connecting the 1.00...
Questions

History, 20.08.2019 09:30

Mathematics, 20.08.2019 09:30

Mathematics, 20.08.2019 09:30


Mathematics, 20.08.2019 09:30

Mathematics, 20.08.2019 09:30

History, 20.08.2019 09:30


Social Studies, 20.08.2019 09:30


Geography, 20.08.2019 09:30


Geography, 20.08.2019 09:30

Mathematics, 20.08.2019 09:30


Social Studies, 20.08.2019 09:30

Mathematics, 20.08.2019 09:30



History, 20.08.2019 09:30