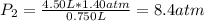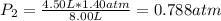Chemistry, 08.12.2020 16:50 itssergioa
The air in a 4.50 L tank has a pressure of 1.40 atm . What is the final pressure, in atmospheres, when the air is placed in tanks that have the following volumes, if there is no change in temperature and amount of gas?
a. 1.00 L
b. 2500. mL
c. 750. mL
d. 8.00 L

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1
You know the right answer?
The air in a 4.50 L tank has a pressure of 1.40 atm . What is the final pressure, in atmospheres, wh...
Questions

English, 10.02.2021 19:20

Mathematics, 10.02.2021 19:20



Mathematics, 10.02.2021 19:20

Mathematics, 10.02.2021 19:20

Mathematics, 10.02.2021 19:20


Mathematics, 10.02.2021 19:20




Mathematics, 10.02.2021 19:20


Mathematics, 10.02.2021 19:20



Mathematics, 10.02.2021 19:20


History, 10.02.2021 19:20