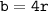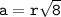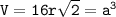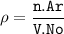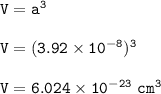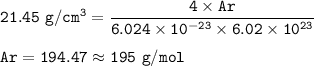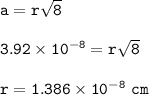Chemistry, 08.12.2020 20:30 nadiarose6345
a metallic object with atoms in a face-centered cubic unit cell with an edge length of 392 pm has a density of 21.45 g/cm^3. calculate the atomic mass and radius of the metal. identify the metal.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
a metallic object with atoms in a face-centered cubic unit cell with an edge length of 392 pm has a...
Questions

Mathematics, 29.06.2021 17:30





Advanced Placement (AP), 29.06.2021 17:30


Mathematics, 29.06.2021 17:30

Social Studies, 29.06.2021 17:40


Social Studies, 29.06.2021 17:40


English, 29.06.2021 17:40





Mathematics, 29.06.2021 17:40

World Languages, 29.06.2021 17:40

Chemistry, 29.06.2021 17:40