
Chemistry, 08.12.2020 22:00 yzafer3971
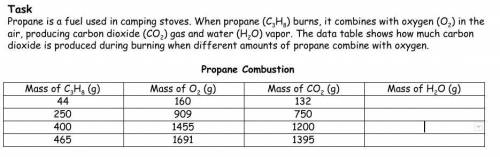
Calculate the mass of water produced and enter in the data table above. Using the Law of Conservation of Matter, Provide a reason explaining the claim and evidence above by showing an example calculation below.

Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:04
The air exhaled from the lungs of a smoker has a concentration of 24 ppm co. express the concentration as a percent.
Answers: 1

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

You know the right answer?
Calculate the mass of water produced and enter in the data table above. Using the Law of Conservatio...
Questions

English, 29.09.2021 17:20


Mathematics, 29.09.2021 17:20

Mathematics, 29.09.2021 17:20



Mathematics, 29.09.2021 17:20




English, 29.09.2021 17:20

English, 29.09.2021 17:20

English, 29.09.2021 17:20



Social Studies, 29.09.2021 17:20

Mathematics, 29.09.2021 17:20

English, 29.09.2021 17:20

Computers and Technology, 29.09.2021 17:20





