
Chemistry, 08.12.2020 22:40 lopezhailey317
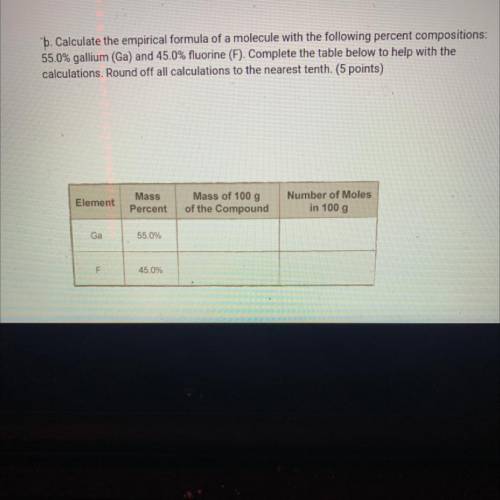
B. Calculate the empirical formula of a molecule with the following percent compositions:
55.0% gallium (Ga) and 45.0% fluorine (F). Complete the table below to help with the
calculations. Round off all calculations to the nearest tenth. (5 points)
Element
Mass
Percent
Mass of 100 g
of the Compound
Number of Moles
in 100 g
Ga
55.0%
F
45.0%

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
B. Calculate the empirical formula of a molecule with the following percent compositions:
55.0% gal...
Questions






Biology, 18.06.2020 15:57








Computers and Technology, 18.06.2020 15:57









