
Chemistry, 08.12.2020 23:40 snowprincess99447
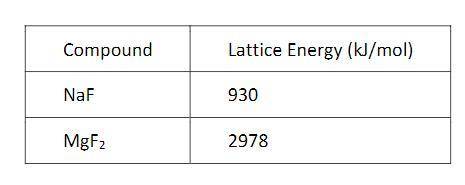
The energy required to dissociate an ionic solid into gaseous ions (lattice energy) for the compounds NaF and MgF2 are shown in the table above. On the basis of Coulomb’s law, which of the following best helps to explain the large difference between the lattice energies of NaF and MgF2?
a. The solubility of MgF2 is less than that of NaF.
b. The electronegativity of Mg is greater than that of Na.
c. The mass of the Mg cation is greater than that of the Na cation.
d. The charge of the Mg cation is larger than that of the Na cation.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
What is the molar mass of babr2? a. 217.2 g/mol b. 297.1 g/mol c. 354.5 g/mol d. 434.4 g/mol
Answers: 1

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
The energy required to dissociate an ionic solid into gaseous ions (lattice energy) for the compound...
Questions



Mathematics, 17.09.2021 14:00

English, 17.09.2021 14:00


Mathematics, 17.09.2021 14:00


Mathematics, 17.09.2021 14:00

History, 17.09.2021 14:00


Mathematics, 17.09.2021 14:00

Computers and Technology, 17.09.2021 14:00


Physics, 17.09.2021 14:00

Biology, 17.09.2021 14:00



Social Studies, 17.09.2021 14:00

English, 17.09.2021 14:00

English, 17.09.2021 14:00



