
Chemistry, 09.12.2020 01:20 amulets8409
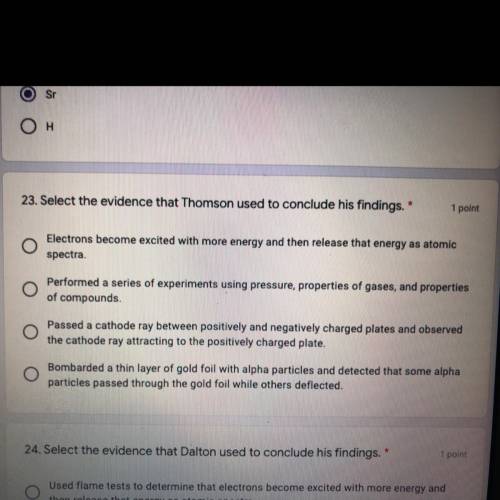
23. Select the evidence that Thomson used to conclude his findings.
(a) Electrons become excited with more energy and then release that energy as atomic
spectra.
(b) Performed a series of experiments using pressure, properties of gases, and properties
of compounds.
(c) Battery Passed a cathode ray between positively and negatively charged plates and observed the cathode ray attracting to the positively charged plate.
(d) Bombarded a thin layer of gold foil with alpha particles and detected that some alpha
particles passed through the gold foil while others deflected. i

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

You know the right answer?
23. Select the evidence that Thomson used to conclude his findings.
(a) Electrons become excited wi...
Questions

English, 14.07.2020 19:01





Mathematics, 14.07.2020 19:01

Mathematics, 14.07.2020 19:01

Social Studies, 14.07.2020 19:01






Mathematics, 14.07.2020 19:01


Mathematics, 14.07.2020 19:01



Mathematics, 14.07.2020 19:01

English, 14.07.2020 19:01



