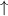Chemistry, 09.12.2020 04:50 cherylmorton7302
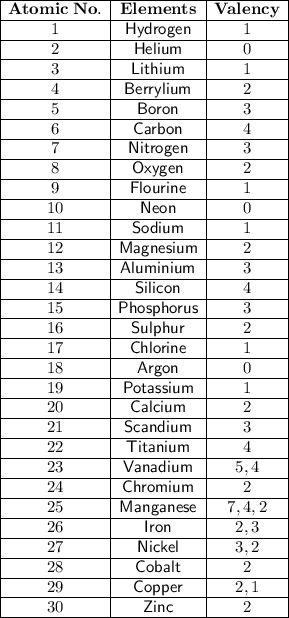
What is the final temperature in degree C when 8.70 grams of Lithium sulfate are dissolved in 60.00 grams of H2O?
Report the temperature to the tenths of a degree.
Heat of solution for Lithium sulfate is - 29.8 kJ / mole. (negative 29.8)
The initial temperature of the water and the Lithium sulfate are both 25.0 oC.
Assume the specific heat of the solution is the same as the specific heat of pure water.
Assume no heat is lost or gained from the surroundings.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1
You know the right answer?
What is the final temperature in degree C when 8.70 grams of Lithium sulfate are dissolved in 60.00...
Questions

Mathematics, 17.02.2021 23:10




Biology, 17.02.2021 23:10

Arts, 17.02.2021 23:10




History, 17.02.2021 23:10


History, 17.02.2021 23:10

Mathematics, 17.02.2021 23:10

Mathematics, 17.02.2021 23:10

Mathematics, 17.02.2021 23:10


Mathematics, 17.02.2021 23:10


Mathematics, 17.02.2021 23:10

Mathematics, 17.02.2021 23:10