
Chemistry, 09.12.2020 21:40 arielcainess
A 62.9 g sample of ground water is found to have a lead concentration of 13.97 ppm. Calculate the mass of lead in the sample in milligrams.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Acetic acid, hc2h3o2, dissolves and completely dissociates in water and a solvation sphere of water molecules forms around the ions. this solute-solvent interaction
Answers: 1

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2
You know the right answer?
A 62.9 g sample of ground water is found to have a lead concentration of 13.97 ppm. Calculate the ma...
Questions


Business, 17.11.2019 23:31


English, 17.11.2019 23:31



Chemistry, 17.11.2019 23:31


Biology, 17.11.2019 23:31




History, 17.11.2019 23:31

History, 17.11.2019 23:31




Health, 17.11.2019 23:31


English, 17.11.2019 23:31

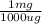 = 0.879 mg of lead
= 0.879 mg of lead


