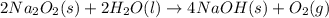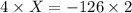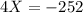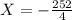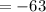Chemistry, 09.12.2020 22:50 cmflores3245
The value of ΔH° for the reaction below is -126 kJ. kj are released when 2.00 mol of NaOH is formed in the reaction?
2 Na2O2 (s) + 2 H2O (l) → 4NaOH (s) + O2 (g)
A) 252
B) 63
C) 3.9
D) 7.8
E) -126

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

You know the right answer?
The value of ΔH° for the reaction below is -126 kJ. kj are released when 2.00 mol of NaOH is formed...
Questions






Mathematics, 22.05.2020 16:57

Mathematics, 22.05.2020 16:57

English, 22.05.2020 16:57

Mathematics, 22.05.2020 16:57


Mathematics, 22.05.2020 16:57

History, 22.05.2020 16:57

Spanish, 22.05.2020 16:57

History, 22.05.2020 16:57


Mathematics, 22.05.2020 16:57



Mathematics, 22.05.2020 16:57

Chemistry, 22.05.2020 16:57