
Chemistry, 09.12.2020 23:40 NetherisIsTheQueen
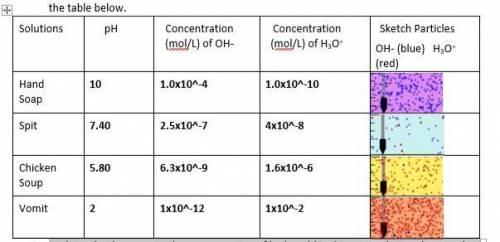
2. Explain what happens to the concentration of hydroxyl (OH-) ions as solutions become less basic.
NO NONSENSE ANSWERS (39 POINTS)
nonsense answers= your mom is _. you fill in the blank

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

You know the right answer?
2. Explain what happens to the concentration of hydroxyl (OH-) ions as solutions become less basic....
Questions

Computers and Technology, 04.05.2021 15:40

Mathematics, 04.05.2021 15:40

Mathematics, 04.05.2021 15:40

Computers and Technology, 04.05.2021 15:40

Mathematics, 04.05.2021 15:40


Biology, 04.05.2021 15:50


Mathematics, 04.05.2021 15:50







English, 04.05.2021 15:50

Computers and Technology, 04.05.2021 15:50

Mathematics, 04.05.2021 15:50




