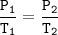Chemistry, 10.12.2020 01:00 chinyere614
The gases in a hairspray can are at a temperature of 27 C and a pressure of 30 psi. if the gases can reach a pressure of 90 psi, the can will explode. To what Temperature must the gases be raised in order for the can to explode? You can assume volume remains constant please help!!!

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
The gases in a hairspray can are at a temperature of 27 C and a pressure of 30 psi. if the gases can...
Questions


Mathematics, 22.11.2020 03:10



Mathematics, 22.11.2020 03:10

Law, 22.11.2020 03:10


English, 22.11.2020 03:10


Chemistry, 22.11.2020 03:10


French, 22.11.2020 03:10

Health, 22.11.2020 03:10

Business, 22.11.2020 03:10

Mathematics, 22.11.2020 03:10

Mathematics, 22.11.2020 03:10

Arts, 22.11.2020 03:10

SAT, 22.11.2020 03:10

Mathematics, 22.11.2020 03:10