Ent will
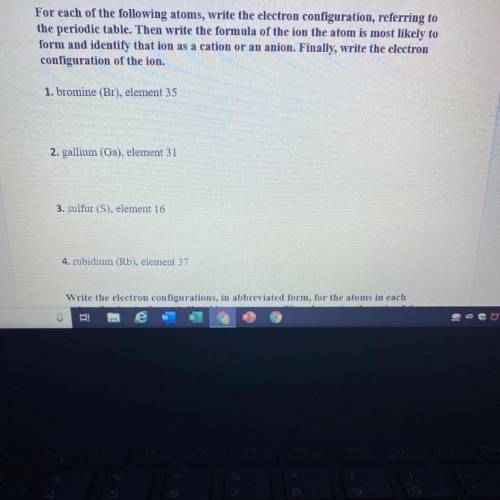
For each of the following atoms, write the electron configuration, referring to
the...

Chemistry, 10.12.2020 01:20 hebibova2016
Ent will
For each of the following atoms, write the electron configuration, referring to
the periodic table. Then write the formula of the ion the atom is most likely to
form and identify that ion as a cation or an anion. Finally, write the electron
configuration of the ion.
1. bromine (Br), element 35
2. gallium (Ga), element 31
3. sulfur (S), element 16
4. rubidium (Rb), element 37
Write the electron con
abbreviated

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
Questions


Chemistry, 23.03.2021 01:00



Chemistry, 23.03.2021 01:00






History, 23.03.2021 01:00


Chemistry, 23.03.2021 01:00

Computers and Technology, 23.03.2021 01:00


Mathematics, 23.03.2021 01:00


History, 23.03.2021 01:00




