Balance the following equation: C7H16 (1) + O2 (g) → CO2 (g) + H2O (g)
...

Chemistry, 10.12.2020 01:20 timmonskids2681
Balance the following equation: C7H16 (1) + O2 (g) → CO2 (g) + H2O (g)
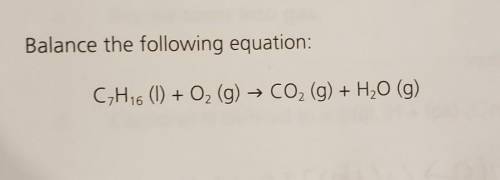

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
You know the right answer?
Questions

Arts, 24.07.2020 09:01

Mathematics, 24.07.2020 09:01

Mathematics, 24.07.2020 09:01

Spanish, 24.07.2020 09:01

Mathematics, 24.07.2020 09:01



Mathematics, 24.07.2020 09:01


Mathematics, 24.07.2020 09:01

English, 24.07.2020 09:01

Mathematics, 24.07.2020 09:01



Mathematics, 24.07.2020 09:01


Mathematics, 24.07.2020 09:01



Computers and Technology, 24.07.2020 09:01



