
Chemistry, 10.12.2020 01:40 angelmosby9
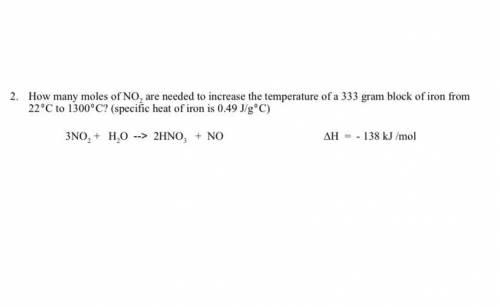
How many moles of NO2 are needed to increase the temperature of a 333 gram block of iron from 220C to 13000C? (specific heat of iron is 0.49 J/g0C) 3NO2 + H2O --> 2HNO3 + NO ∆H = - 138 kJ /mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
How many moles of NO2 are needed to increase the temperature of a 333 gram block of iron from 220C t...
Questions

Mathematics, 25.10.2020 02:50


Mathematics, 25.10.2020 02:50




History, 25.10.2020 02:50


Mathematics, 25.10.2020 02:50

Mathematics, 25.10.2020 02:50


Law, 25.10.2020 02:50

Mathematics, 25.10.2020 02:50

History, 25.10.2020 02:50

Mathematics, 25.10.2020 02:50

Mathematics, 25.10.2020 02:50


Mathematics, 25.10.2020 02:50

History, 25.10.2020 02:50

Mathematics, 25.10.2020 02:50



