
Chemistry, 10.12.2020 04:00 lizzyhearts
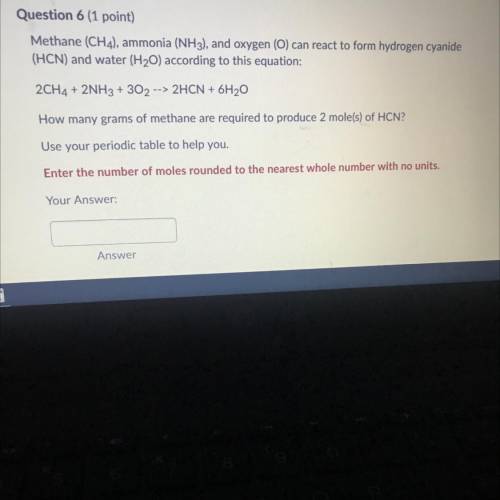
Methane (CH 4 ) , ammonia (NH 3 ) , and oxygen () can reaci to form hydrogen cyanide (HCN) and water (H 2 O) according to this equation 2CH 4 +2NH 3 +3O 2 2HCN+6H 2 O How many grams of methane are required to produce 2 moles ? Use your periodic table to help you. Please show work

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
You know the right answer?
Methane (CH 4 ) , ammonia (NH 3 ) , and oxygen () can reaci to form hydrogen cyanide (HCN) and water...
Questions



Mathematics, 03.09.2020 20:01

Mathematics, 03.09.2020 20:01



English, 03.09.2020 20:01


Social Studies, 03.09.2020 20:01

Biology, 03.09.2020 20:01


English, 03.09.2020 20:01

Social Studies, 03.09.2020 20:01



Mathematics, 03.09.2020 20:01

Biology, 03.09.2020 20:01


Geography, 03.09.2020 20:01

Chemistry, 03.09.2020 20:01



