
Chemistry, 10.12.2020 06:20 jonathon3957
URGENT PLEASE HELP!
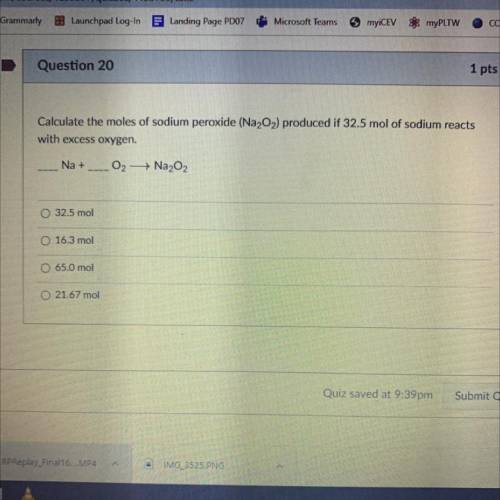
Calculate the moles of Sodium Peroxide (Na2O2) produced if 32.5 mol of sodium reacts with excess oxygen.
__Na+__O2—>Na2O2
A. 32.5 mol
B. 16.3 mol
C. 65.0 mol
D. 21.67 mol
and why is it one of the answer above?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
URGENT PLEASE HELP!
Calculate the moles of Sodium Peroxide (Na2O2) produced if 32.5 mol of sodium r...
Questions

History, 27.10.2020 23:00

Mathematics, 27.10.2020 23:00


English, 27.10.2020 23:00

English, 27.10.2020 23:00


Mathematics, 27.10.2020 23:00



Mathematics, 27.10.2020 23:00


Health, 27.10.2020 23:00

Mathematics, 27.10.2020 23:00





Mathematics, 27.10.2020 23:00




