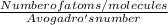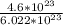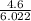Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3
You know the right answer?
How many moles are in 4.6x1023 molecules of H20 expressed in the correct number
of significant figu...
Questions

Mathematics, 11.11.2019 01:31




Mathematics, 11.11.2019 01:31




English, 11.11.2019 01:31



Biology, 11.11.2019 01:31

English, 11.11.2019 01:31

History, 11.11.2019 01:31





Mathematics, 11.11.2019 01:31

Mathematics, 11.11.2019 01:31