

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
This graph gives information on changes in sea ice extent in the arctic ocean over a 30-year span. the overall trend shows in the ice extent. to address the trend, scientists need to ask themselves, one direct consequence of the trend is that
Answers: 1

Chemistry, 21.06.2019 17:30
When the earth was formed and cooled, why did nickel and iron end up in the center of the earth while basalt and granite ended up in the outer layers
Answers: 3

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1
You know the right answer?
Calculate the maximum volume in mL of 0.20 M HCl that a tablet containing 338 mg Al(OH)3 and 489 mg...
Questions

Mathematics, 04.12.2020 22:30


Mathematics, 04.12.2020 22:30



Biology, 04.12.2020 22:30

Mathematics, 04.12.2020 22:30



Social Studies, 04.12.2020 22:30

Mathematics, 04.12.2020 22:30



Social Studies, 04.12.2020 22:30



Chemistry, 04.12.2020 22:30



Computers and Technology, 04.12.2020 22:30

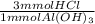 = 13 mmol HCl
= 13 mmol HCl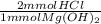 = 16.77 mmol HCl
= 16.77 mmol HCl

