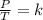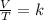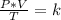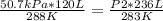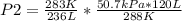Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1
You know the right answer?
At 50.7 kPa and 15.0 °C a sample of gas occupies 120 L. Using the Combined Gas Law, what pressure do...
Questions



Mathematics, 28.08.2019 22:00

History, 28.08.2019 22:00



History, 28.08.2019 22:00



Social Studies, 28.08.2019 22:00


History, 28.08.2019 22:00


Mathematics, 28.08.2019 22:00

History, 28.08.2019 22:00


English, 28.08.2019 22:00

Mathematics, 28.08.2019 22:00


Biology, 28.08.2019 22:00