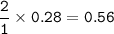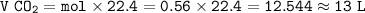HELP FOR FINAL
In an industrial process ethanol C2H6o burns with Oz to produce heat.
C2H5OH +...

Chemistry, 10.12.2020 19:50 anthonybowie99
HELP FOR FINAL
In an industrial process ethanol C2H6o burns with Oz to produce heat.
C2H5OH + 3 o2 - 2 CO2 + 3 H20 + 8842 Joules
How many liters of CO2 are obtained from burning 0.28 moles of ethanol at STP (standard temperature and pressure? Express your result with 2 significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
You know the right answer?
Questions

Mathematics, 02.11.2020 21:20

English, 02.11.2020 21:20

Mathematics, 02.11.2020 21:20


Mathematics, 02.11.2020 21:20

Physics, 02.11.2020 21:20


Mathematics, 02.11.2020 21:20


Biology, 02.11.2020 21:20

History, 02.11.2020 21:20

Arts, 02.11.2020 21:20

Biology, 02.11.2020 21:20

Computers and Technology, 02.11.2020 21:20

Mathematics, 02.11.2020 21:20

Mathematics, 02.11.2020 21:20

Biology, 02.11.2020 21:20

English, 02.11.2020 21:20

English, 02.11.2020 21:20

Mathematics, 02.11.2020 21:20