
Chemistry, 10.12.2020 20:50 YaBoiMando2061
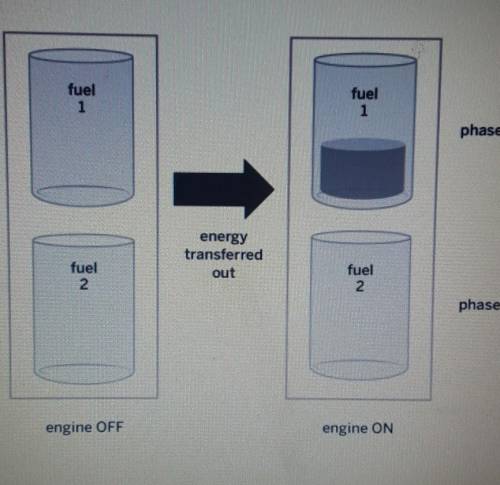
PLEASE ANSWER A certain type of ship has two tanks in its engine. Each tank contains a different type of fuel. When the engine turns on, the same amount of energy is transferred out of both fuels as shown in the diagram below. Why did fuell change phase. but fuel 2 stayed the same? Explain what happened to the molecules of both fuels.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
PLEASE ANSWER
A certain type of ship has two tanks in its engine. Each tank contains a different ty...
Questions


Advanced Placement (AP), 02.02.2021 01:00

Health, 02.02.2021 01:00


Mathematics, 02.02.2021 01:00


Mathematics, 02.02.2021 01:00

Mathematics, 02.02.2021 01:00



Chemistry, 02.02.2021 01:00

Mathematics, 02.02.2021 01:00




Mathematics, 02.02.2021 01:00

History, 02.02.2021 01:00

Social Studies, 02.02.2021 01:00




