
A concept map for four types of intermolecular forces and a certain type of bond is shown.
Which of the following correctly identifies the intermolecular force represented by A and compares its strength relative to the intermolecular force represented by b.
1. A represents London dispersion forces, which are weaker than the force represented by b
2. A represents hydrogen bonding, which is weaker than the force represented by b
3. A represents London dispersion forces, which are stronger than the force represented by b
4. A represents hydrogen bonding, which is stronger than the force represented by b
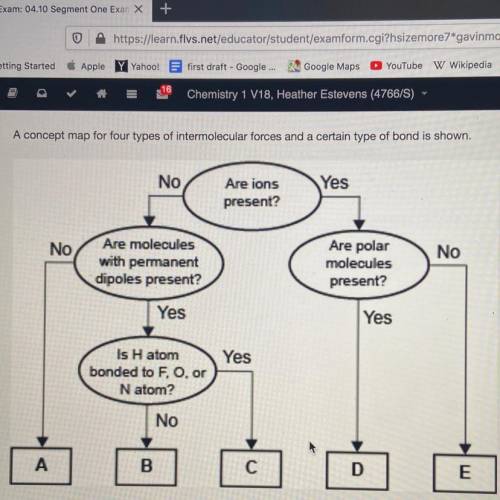

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:50
How do the energy differences between the higher energy levels of an atom compare with the energy difference between the lower energy level of the atom
Answers: 1

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

You know the right answer?
A concept map for four types of intermolecular forces and a certain type of bond is shown.
Which of...
Questions

Mathematics, 27.10.2020 20:10


Mathematics, 27.10.2020 20:10

Social Studies, 27.10.2020 20:10

Mathematics, 27.10.2020 20:10


Mathematics, 27.10.2020 20:10

History, 27.10.2020 20:10



Mathematics, 27.10.2020 20:10

Business, 27.10.2020 20:10

Biology, 27.10.2020 20:10

Biology, 27.10.2020 20:10

Mathematics, 27.10.2020 20:10

Mathematics, 27.10.2020 20:10

History, 27.10.2020 20:10

Medicine, 27.10.2020 20:10

Mathematics, 27.10.2020 20:10

Mathematics, 27.10.2020 20:10



