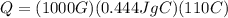Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2



Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
A 1000-g iron frying pan is placed on a stove. The pan increases from 20°C to 130°C. If the specific...
Questions


Mathematics, 12.12.2019 21:31


History, 12.12.2019 21:31


Biology, 12.12.2019 21:31

English, 12.12.2019 21:31

Social Studies, 12.12.2019 21:31


English, 12.12.2019 21:31




Mathematics, 12.12.2019 21:31

Social Studies, 12.12.2019 21:31

Social Studies, 12.12.2019 21:31

Social Studies, 12.12.2019 21:31

History, 12.12.2019 21:31

Chemistry, 12.12.2019 21:31