
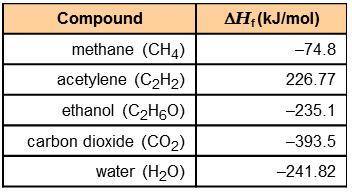
Using the information in the table to the right, calculate the enthalpy of combustion of each of the following substances:
acetylene:
ethanol:
The combustion of 0.25 mol of an unknown organic compound results in the release of 320 kJ of energy. Which of the compounds in the table could be the unknown compound?
ANSWERS:
1.
acetylene: -1,256 kJ/mol
ethanol: -1,277 kJ/mol
2.
ethanol
I already know the answers (they're right there ^) i just need to know HOW you find the enthalpy combustion of acetylene and ethanol.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
Using the information in the table to the right, calculate the enthalpy of combustion of each of the...
Questions


World Languages, 25.08.2019 01:30



Mathematics, 25.08.2019 01:30


Mathematics, 25.08.2019 01:30



Mathematics, 25.08.2019 01:30

History, 25.08.2019 01:30


Mathematics, 25.08.2019 01:30


Mathematics, 25.08.2019 01:30


Physics, 25.08.2019 01:30


Mathematics, 25.08.2019 01:30

Business, 25.08.2019 01:30



