Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2
You know the right answer?
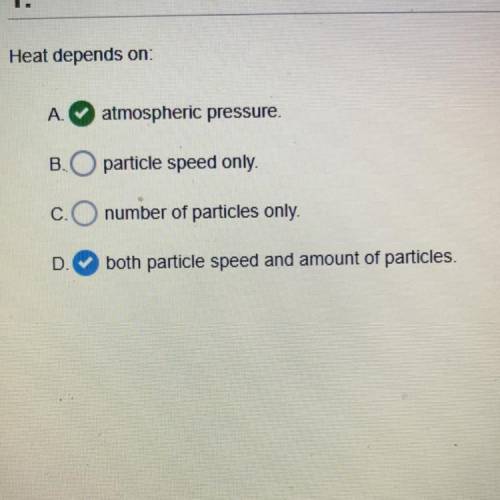
Heat depends on A. Atmospheric pressure B. Particle speed only C. Number of particles D. Both speed...
Questions

Mathematics, 23.04.2021 23:10

English, 23.04.2021 23:10

History, 23.04.2021 23:10




Chemistry, 23.04.2021 23:10



Mathematics, 23.04.2021 23:10

Mathematics, 23.04.2021 23:10

Biology, 23.04.2021 23:10

Mathematics, 23.04.2021 23:10

Mathematics, 23.04.2021 23:10


Mathematics, 23.04.2021 23:10

History, 23.04.2021 23:10

Mathematics, 23.04.2021 23:10