
Chemistry, 12.12.2020 16:30 animaljamissofab
A. What is the atomic number for this particle
B. What is the mass number of the particle
C. what is the overall charge on this particle
D. What element does this particle represent
E. Is this particle an atom or an ion
F. Write the symbol that represents this particle. Include the symbol, mass number, atomic number and charge
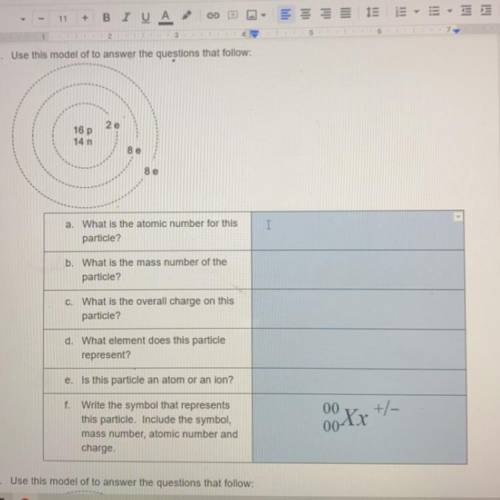

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
A. What is the atomic number for this particle
B. What is the mass number of the particle
Questions



Chemistry, 06.03.2020 21:43






Advanced Placement (AP), 06.03.2020 21:43






English, 06.03.2020 21:43


Mathematics, 06.03.2020 21:43


Mathematics, 06.03.2020 21:43



