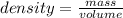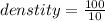Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Which of these will change if the air in aclosed bottle is heated? abcdthe mass of the airthe composition of the airthe air pressure in the bottlethe number of air molecules in the bottle
Answers: 3

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
A substance has a mass of 100 g and a volume of 10 mL. What is the
density of the substance? *
Questions

Mathematics, 20.02.2021 15:30

Mathematics, 20.02.2021 15:30


Mathematics, 20.02.2021 15:30

English, 20.02.2021 15:30

Chemistry, 20.02.2021 15:30


Mathematics, 20.02.2021 15:30

Mathematics, 20.02.2021 15:30


Mathematics, 20.02.2021 15:30




Chemistry, 20.02.2021 15:40

Mathematics, 20.02.2021 15:40

Health, 20.02.2021 15:40

Physics, 20.02.2021 15:40


Mathematics, 20.02.2021 15:40