
Chemistry, 12.12.2020 16:50 yarbor800592
Hydrazine reacts with oxygen according to the following equation: N2H4(g) +O2(g) → N2(g) + 2 H2O(l) How many L of N2, measured at 34.9 °C and 755.08 torr, will be produced at the same time that 914.894 g of H2O is produced?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

You know the right answer?
Hydrazine reacts with oxygen according to the following equation: N2H4(g) +O2(g) → N2(g) + 2 H2O(l)...
Questions

English, 11.10.2021 19:10



Mathematics, 11.10.2021 19:20

Mathematics, 11.10.2021 19:20





Mathematics, 11.10.2021 19:20

Mathematics, 11.10.2021 19:20

English, 11.10.2021 19:20

Biology, 11.10.2021 19:20

Mathematics, 11.10.2021 19:20



Mathematics, 11.10.2021 19:20


History, 11.10.2021 19:20

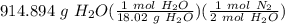 = 25.3955 mol N₂
= 25.3955 mol N₂

