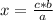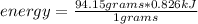Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2
You know the right answer?
The heat of vaporization for ethanol is 0.826 kJ/g. Calculate the heat energy in joules required to...
Questions



Geography, 04.11.2021 14:00


Mathematics, 04.11.2021 14:00


Mathematics, 04.11.2021 14:00




Business, 04.11.2021 14:00


Mathematics, 04.11.2021 14:00



Arts, 04.11.2021 14:00

English, 04.11.2021 14:00

Arts, 04.11.2021 14:00