
Chemistry, 12.12.2020 17:10 janayshas84
What amount of the excess reagent remains when 0.30 mol NH3 reacts with 0.40 mol O2 to produce NO and H2O? 4NH3 +502 + 4NO + 6H20
(A) 0.10 mol NH,
(B) 0.10 mol
(C) 0.025 mol NH
(D) 0.025 mol O2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3


Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
What amount of the excess reagent remains when 0.30 mol NH3 reacts with 0.40 mol O2 to produce NO an...
Questions

History, 25.06.2019 02:30

Mathematics, 25.06.2019 02:30

Physics, 25.06.2019 02:30

History, 25.06.2019 02:30

English, 25.06.2019 02:30


History, 25.06.2019 02:30




History, 25.06.2019 02:30

Mathematics, 25.06.2019 02:30

Spanish, 25.06.2019 02:30



Biology, 25.06.2019 02:30

Mathematics, 25.06.2019 02:30

Mathematics, 25.06.2019 02:30

English, 25.06.2019 02:30

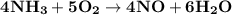
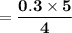
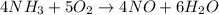
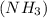 reacts with = 5 moles of oxygen
reacts with = 5 moles of oxygen 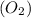
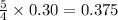 moles of oxygen
moles of oxygen 



