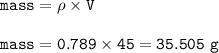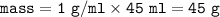Answers: 2


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Asample of rose gold is: 12.0 g gold, 5.0g silver, and 7.0 g copper. what is the percent copper in the sample?
Answers: 2

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2
You know the right answer?
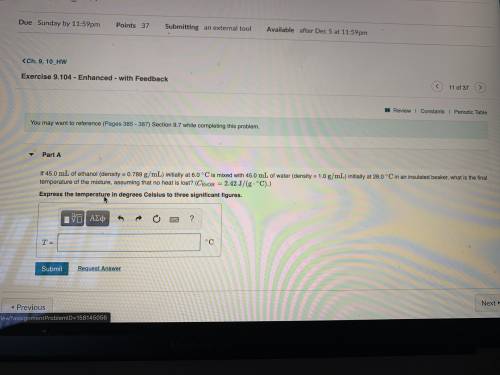
If 45.0 mL of ethanol (density =0.789g/mol) initially at 6.0°C mix with 45.0 mL of water (density =1...
Questions

Mathematics, 12.03.2021 02:30


Mathematics, 12.03.2021 02:30

Mathematics, 12.03.2021 02:30

Chemistry, 12.03.2021 02:30

Mathematics, 12.03.2021 02:30






History, 12.03.2021 02:30

Mathematics, 12.03.2021 02:30

English, 12.03.2021 02:30



Arts, 12.03.2021 02:30



Mathematics, 12.03.2021 02:30