
Chemistry, 13.12.2020 19:40 pizzaboy62
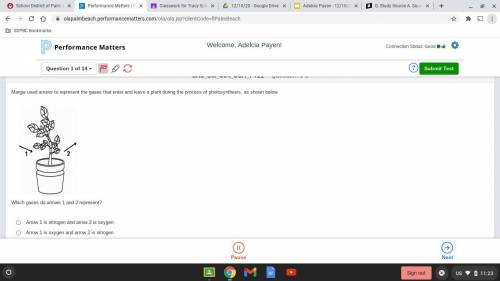
Please help i know it's a test the answers: A. Arrow 1 is nitrogen and arrow 2 is oxygen. B. Arrow 1 is oxygen and arrow 2 is nitrogen. C. Arrow 1 is oxygen and arrow 2 is carbon dioxide. D. Arrow 1 is carbon dioxide and arrow 2 is oxygen. show proof

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉more accurate estimates can be made with the van der waals equationí‘ťí‘ť=푛푛푛푛푛푛푉푉â’푛푛푟푟â’푞푞푛푛2푉푉2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
Please help i know it's a test
the answers: A. Arrow 1 is nitrogen and arrow 2 is oxygen. B. Arrow...
Questions

Mathematics, 01.04.2021 22:30



English, 01.04.2021 22:30






Mathematics, 01.04.2021 22:30

Mathematics, 01.04.2021 22:30

Mathematics, 01.04.2021 22:30


Mathematics, 01.04.2021 22:30

Advanced Placement (AP), 01.04.2021 22:30

History, 01.04.2021 22:30

Mathematics, 01.04.2021 22:30

Spanish, 01.04.2021 22:30


Mathematics, 01.04.2021 22:30



