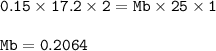Chemistry, 13.12.2020 23:20 bekahmc1p6k6vj
A student used a pipette to add 25.0 cm of KOH of unknown concentration to
a conical flask.
The student carried out a titration experiment to find the volume of 0.150 mol/dmº
H2SO4 needed to neutralise the KOH.
The student found that, on average, 17.20 cm of the H2SO4 solution was
required for neutralisation.
Calculate the concentration of the KOH solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Litmus paper is made from water-soluble dyes which are extracted from lichens. this paper is used as an acid-base indicator. which of these common household substances would turn blue litmus paper red? a) bleach b) lye c) soap d) vinegar
Answers: 3

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

You know the right answer?
A student used a pipette to add 25.0 cm of KOH of unknown concentration to
a conical flask.
Th...
Th...
Questions



Computers and Technology, 07.12.2021 16:40



Chemistry, 07.12.2021 16:50



Chemistry, 07.12.2021 16:50

Mathematics, 07.12.2021 16:50


Social Studies, 07.12.2021 16:50



English, 07.12.2021 16:50

Physics, 07.12.2021 16:50


English, 07.12.2021 16:50


Mathematics, 07.12.2021 16:50