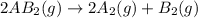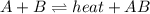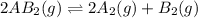1. chemical equilibrium is established when the number of reactants equals the number of products.. - true. - false. 2. according to le chatelier's principle, by increasing the temperature of the system shown below, the equilibrium will shift to the right (towards . a + b heat + ab. - true. - false. 3. for which of the following reactions will an increase in pressure not effect the position of equilibrium? . . a. 2a2 (g) + b2 (g) 2a2b (g). b. 2ab (g) a2 (g) + b2 (g). c. 2a (g) + f2 (g) 2af (g). d. 2b (s) + 2ha (aq) 2ba (aq) + h2 (g).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

You know the right answer?
1. chemical equilibrium is established when the number of reactants equals the number of products.....
Questions

Mathematics, 04.05.2021 22:20

Mathematics, 04.05.2021 22:20








Arts, 04.05.2021 22:20



Chemistry, 04.05.2021 22:20

History, 04.05.2021 22:20


Mathematics, 04.05.2021 22:20

Health, 04.05.2021 22:20

Mathematics, 04.05.2021 22:20